Clinical trials, pilot production of the probe and other updates
December 9, 2022
Several intense months have passed since the last update. Our team has been diligently working on the implementation of Phase I of the clinical trials, which began during the summer. The engineers are focused on the pilot production of the inPROBE® microsensor. Quality specialists are overseeing the proper implementation of the ISO system and MDR requirements. At the same time, the company is expanding its international network of contacts and consistently working to maintain interest in inPROBE® both in Poland and abroad. Read the article to find out what else has been happening — and is still happening — in our company.
Clinical trials
One of the most important events of the past weeks at SDS Optic S.A. was undoubtedly the official launch of clinical trials. Phase I began with a study conducted at the Clinic of Oncological Surgery at SPSK 1 in Lublin on August 25, 2022. The trial was preceded by initiation training, during which the medical staff became familiar with the procedures and the operating principles of the inPROBE® technology. The event was announced in the current ESPI report 13/2022.
View the full ESPI report from August 25, 2022Phase I - safety study - 18 female patients with breast cancer HER2+ and HER2-
- •observation of adverse events
- •determination of the feasibility of the inPROBE study
- •preliminary correlation of the measurement and receptor status
The planned completion of research under Phase I is scheduled for December 2022. After this stage concludes and the corresponding final research report is prepared, Phase II will begin. In Phase II, researchers will focus on confirming the safety and the correlation of the HER2 receptor status detected by the probe with the existing diagnostic standard on the market. This will serve as confirmation of the effectiveness of our probe. Five medical institutions will participate in the second part of the clinical trials. The planned duration of this phase is between 6 to 8 months, depending largely on the patient testing schedules at each institution.

The clinical centers have already been verified, and the schedule for initiation training for the medical staff participating in the studies has been planned. An external CRO partner is overseeing the conduct of the clinical trials.
Phase II - Efficacy study – 192 patients with breast cancer
- •confirmation of safety in a larger number of female patients
- •determination of the effectiveness of the diagnostic method
The clinical trial we are conducting are open, multicenter studies evaluating the safety and effectiveness of the diagnostic microsensor (inPROBE®) designed to assess HER2 receptor expression in a population of women at high risk of breast cancer. If the results confirm the initial assumptions and the outcomes of preclinical studies, the Company will be able to proceed with further work related to the commercialization of the technology. The results obtained during the clinical trials will constitute an extremely valuable component of the Company’s know-how. They will also enrich the existing knowledge base regarding the use of photonics and molecular biology in in vivo and real-time diagnostics and health monitoring.
Pilot production
Production independence is one of the main goals we are striving for. It is aimed not only at generating revenue but also at developing ready-made procedures and know-how in the field of mass production of the inPROBE® microsensor. This will constitute an invaluable advantage in the commercialization of the solution. On November 14, 2022, we achieved another milestone by completing the investment phase related to the implementation of pilot production of optoelectronic components for fiber-optic biosensors used in inPROBE® microsensors. The company received the final and crucial piece of specialized equipment from the US, customized for the needs of the planned production of optoelectronic components for fiber-optic biosensors. This marked the completion of the investment phase, which included ordering, purchasing, delivering, and commissioning production equipment and machinery worth PLN 2.4 million. The company financed this milestone with funds obtained from the Series D share issue conducted in 2021.
Our clean room has been equipped with the necessary devices for the planned stage of production of optoelectronic components for fiber-optic biosensors, which we use in inPROBE®microsensors. This is one of the key steps toward achieving production independence on a semi-industrial scale – the planned production capacity of the clean room is approximately 50,000 biosensors per year. We have previously secured the capability to independently produce biological, chemical, and bioengineering components, whereas the fiber-optic biosensors were until now manufactured by external partners. We will soon begin work on calibrating and optimizing the production processes using the purchased machines and equipment. At the same time, we will be conducting a recruitment process for new members of the R&D team. The next step will be to carry out the validation and certification process of the planned production – its completion is expected in Q4 2023. As a result, complete certified process and production documentation will be created. Our potential business partners will thus gain assurance that mass-scale production of the inPROBE® microsensor is possible. We believe this will be a key value at the commercialization stage. - Marcin Staniszewski, CEO of SDS Optic S.A.
Obtained patents
The company has received positive decisions regarding the granting of patents for applications related to key components of our inPROBE® biosensor. The granted patents cover a device for detecting and/or determining the concentration of an analyte present in tissue, as well as a method utilizing this device. This means the company has obtained protection for crucial elements of the optical sensor technology (the inPROBE® fiber-optic microsensor), which enables measurements to be taken in vivo or in isolated tissue, including fixed tissue, without any need for tissue preparation. Our measurement method is fast and sensitive and does not require the use of fluorescent or chemiluminescent markers, which offers an additional safety advantage for both doctors and patients. The patents granted by the US Patent and Trademark Office and the European Patent Office are valid for 20 years from the filing date, i.e., from May 26, 2017. The patent covers the US and 38 countries under the jurisdiction of the EPO.
Distinction for The Best Annual Report 2021 in the NewConnect market
In October 2022, SDS Optic S.A. also received a special award for the best debut in the category of activity reports of companies listed on the NewConnect market. The statuette was accepted on behalf of the Management Board by Karol Maryniowski, who is responsible for investor relations, marketing, and communication at the company. The Best Annual Report is a competition held annually by the Institute of Accounting and Taxes, known for its reliability in analyzing submitted reports and the high level of expertise of its judging panel. The award confirms the quality of the reports delivered to the market and validates the Issuer’s development direction in the broader context of market communication. The award was received on behalf of the company by Karol Maryniowski, Head of Communication, Marketing, and Investor Relations — pictured in the center of the photo.

Presentation of scientific results at the ENA 2022 symposium in Barcelona.
Representatives of the R&D team at SDS Optic S.A. — Magdalena Staniszewska, Scientific Director and Co-founder of the company, and Agata Mitura, Research Team Leader – Biology — participated in the prestigious 34th EORTC-NCI-AACR Symposium held in Barcelona from October 26 to 28, 2022. The company presented the results of its own scientific research, which indicate an accumulation of the soluble form of the HER2 receptor in the area surrounding HER2+ tumors. This finding confirms that it is an optimal site for diagnostics using the inPROBE® sensor. In addition to the poster presentation, the event offered a unique opportunity to explore the latest developments in cancer drug research, the use of liquid biopsy techniques in diagnostics and therapy monitoring, and the legal regulations governing clinical trials. Participation in the symposium and the presentation of research findings to experts from various fields of oncology highlighted new possibilities for the application of inPROBE® technology and potential directions for the future development of our technology.
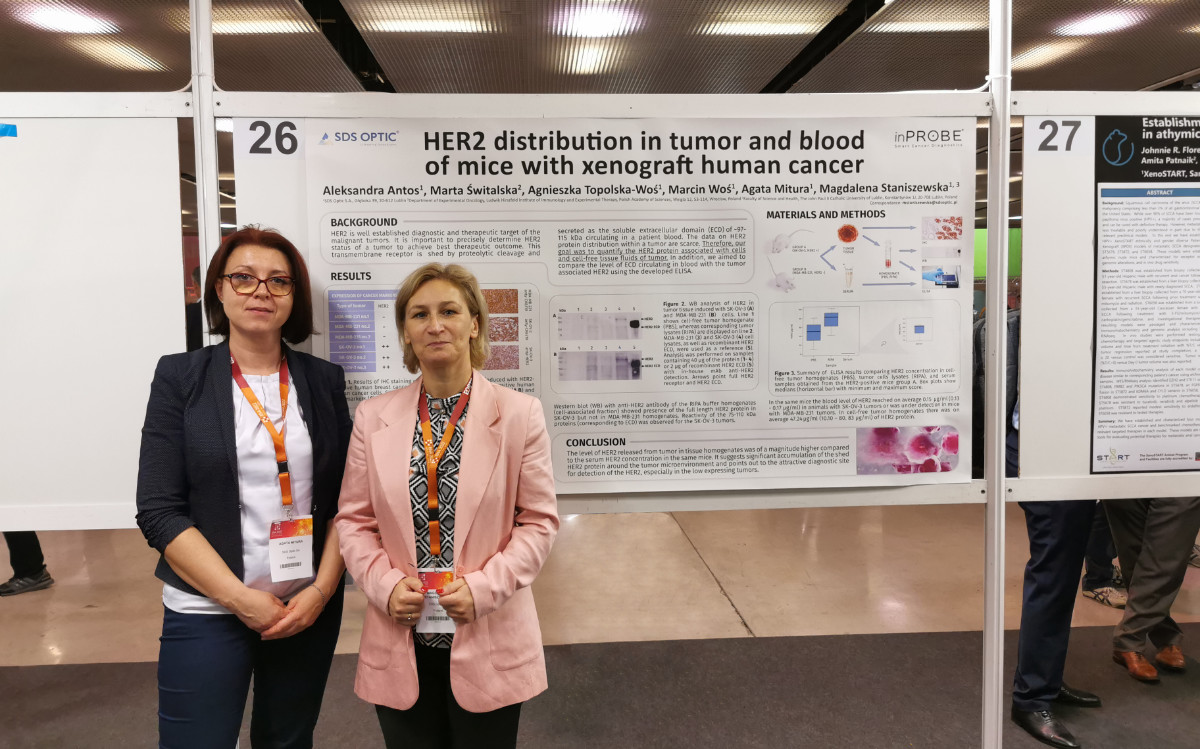
Poster Presented at 34’th EORTC-NCI-AACR, Barcelona 2022, P. session PP02 – Animal Models, p no. 36
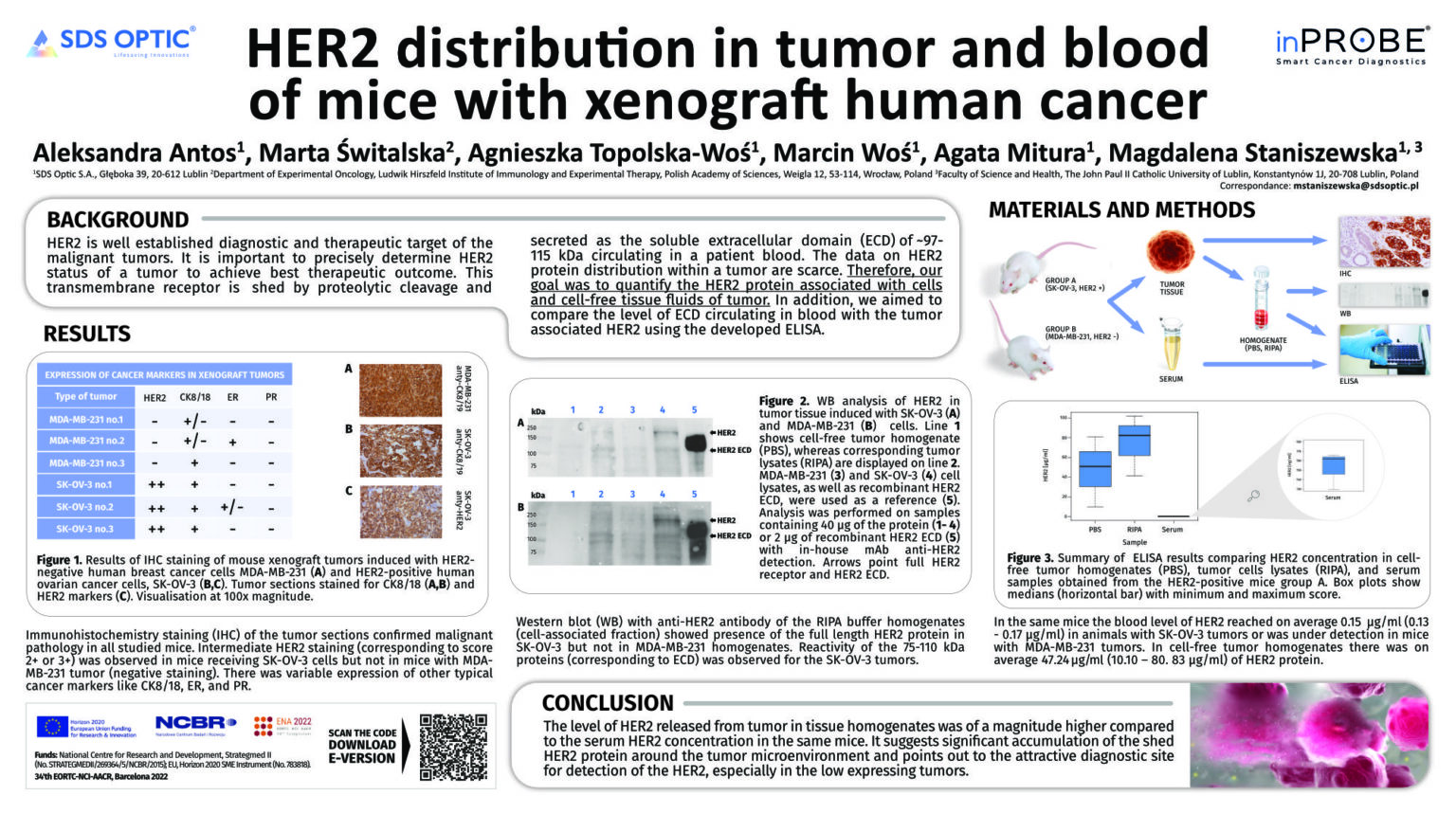 Download the full poster presentation
Download the full poster presentationWe Care About Your Privacy
SDS Optic S.A. uses cookies to improve and customize users experience on our website. By selecting 'Accept', you consent to the use of all cookies that gather and use information about your interactions with our site to provide personalized content and enhance your digital experience. Please read our Privacy Policy for more information.